Electronic Data Capture (EDC) systems are used to collect, store and manage data generated from clinical trials or other research studies in electronic forms which can either replace the traditional paper forms or be used in conjunction with paper forms for which data is then transcribed into the electronic forms. EDC systems are typically used for data collection for clinical trials but Dacima Software also specializes in data collection for other medical research study designs, including observational epidemiological study designs (cohort, case-control, and cross-sectional studies), as well as for post-marketing studies and patient registries.

Modern electronic data capture software applications are typically web-based and utilize a thin client. This allows end users to access the study database through the Web through a web browser without the need for the installation of an application on the local computer.
Dacima Clinical Suite Randomization ePRO/Web survey Patient Registry AEFI
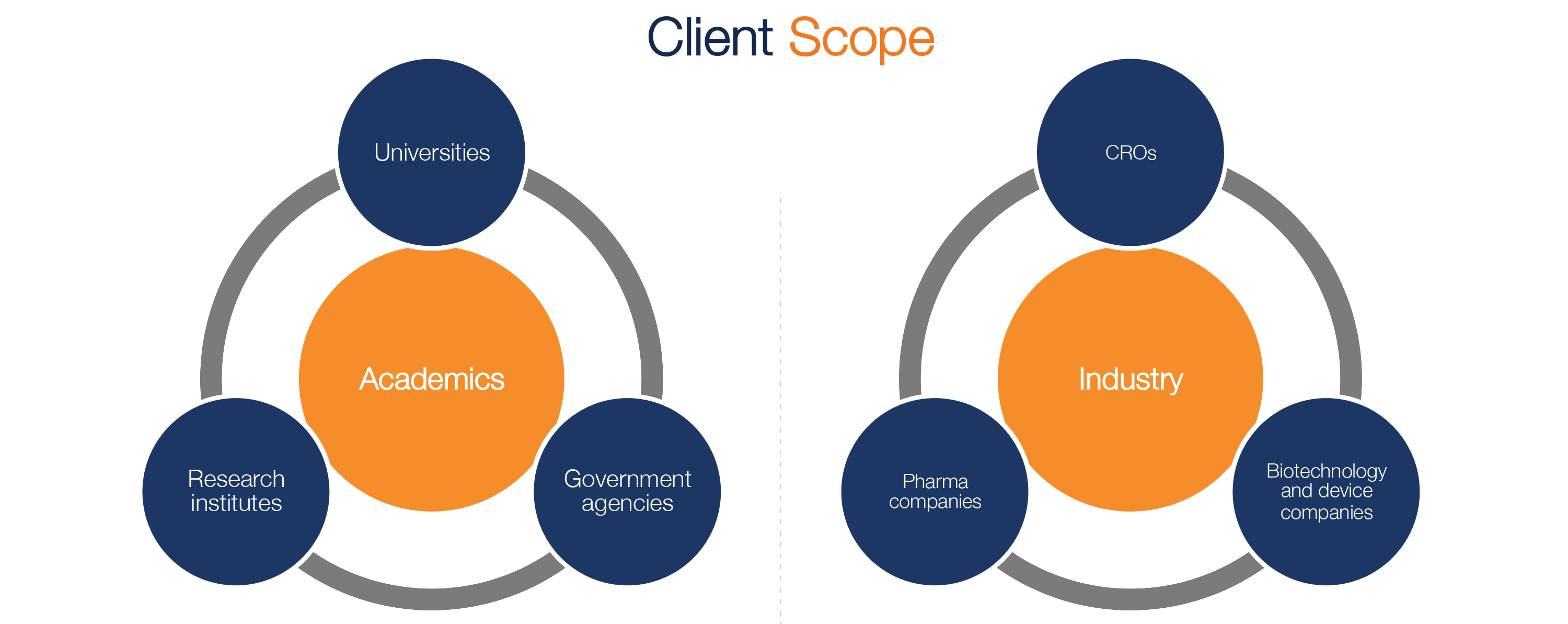
Our EDC software solutions are used by life sciences organizations including academic researchers, research institutions, health networks, government agencies, as well as, Clinical Research Organizations, biotechnology pharmaceutical companies.


Contact us to receive a fully functional trial version of our software including a 30-minutes guide.
@Dacima Software Inc is part of the EvidentIQ Group along with Carenity, Fortress Medical Systems and XClinical