
COLLECT DATA DIRECTLY FROM PATIENTS AT THE RIGHT MOMENT
Enhance patient engagement and simplify diary data collection by using Dacima’s eDiary intuitive web interface.
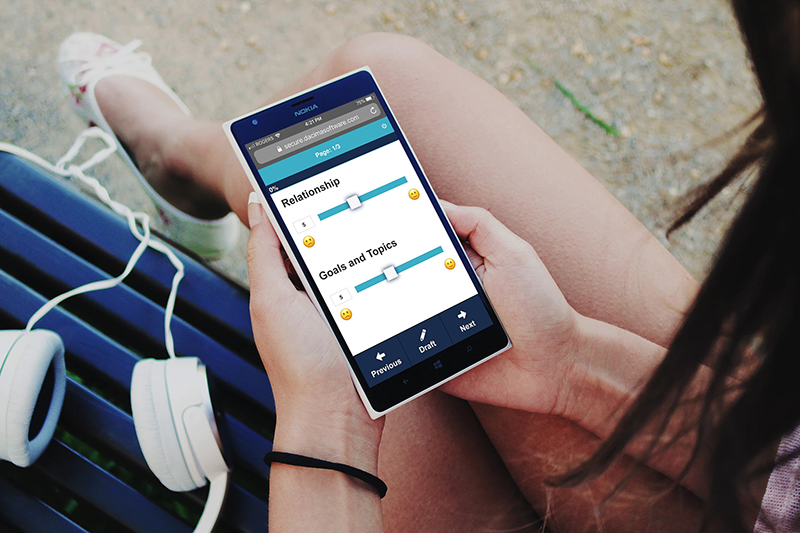

eDIARY
IMPROVE PATIENT ENGAGEMENT AND DATA QUALITY
Our eDiary is an electronic Clinical Outcome Assessment (eCOA) software that supports building online diaries to be completed by participants using a PC, tablet, or smartphone. Our eDiary complies with FDA 21 CFR Part 11, Good Clinical Practices (GCP) and contains a complete audit trail, making it the best choice for medical and health-related research studies.
FEATURES
- Designed to work on a smartphone for easy daily use.
- Integrated with the Visit Scheduler module
- Send out scheduled links for diary completion.
- Supports sending (multiple) reminders (email/SMS).
- Notiy sites if diary expires.
- Customizable invitation emails/SMS.
- Tailor welcome and completion pages to meet your needs
- Track patient completion in custom reports and dashboards.
- Ability to create conditional dependencies to enable fields, to make fields visible or hidden, trigger popup messages and change colors.
- Choose from different data extraction options.
- Use the Query builder to extract subsets of data or generate aggregate and summary statistics reports.
- Build-in reports lets you view summary statistics, generate a data dictionary, data query reports and more.
DIARY MANAGER
This option gives you access to diary management tools for tracking respondent diary completion, diary status, reports, data extraction options, query builder (create custom reports and outputs), managing invitations, reminders, welcome and other pages.

