
ACCELERATE TRIALS AND SIMPLIFY COMPLEXITY
Dacima offers a cutting-edge EDC and CDMS for clinical research data. It captures, manages, and reports on every detail in order to optimize your research data processes. Dacima’s EDC has been designed for the needs of CROs, pharma companies, government agencies, research institutions and universities alike.
Single Platform - Flexible, Scalable, Configurable
Flexible
Scalable
Configurable
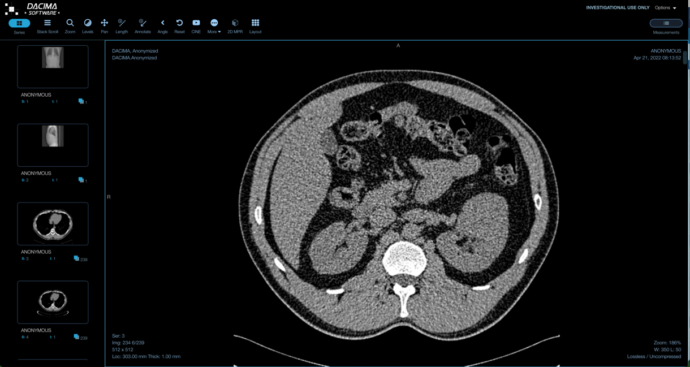
DICOM IMAGE VIEWER
UPLOAD DICOM FORMAT IMAGES INTO CRF
Upload DICOM files and view them with annotation and measurement capabilities. We offer de-identification/anonymization of patient identification information option on upload. Batch extraction of DICOM files (manual or scheduled) extraction to sFTP site.
OFFLINE DATA COLLECTION
MORE POSSIBILITIES IN REMOTE AREAS
The offine module allows data collection in regions with poor or limited internet connectivity. The offline mode combines the benefits of web electronic data capture with that of a distributed offline database system. The system automatically generates a client application from the web database, replicating the interface of web database with all the validation checks, form design features, data querying capabilities and data collection workflow of the original master web database.
Research staff can collect data electronically using the distributed database system without the need for internet access. When arriving at the clinical site/office, the software will synchronize with the online version by comparing it to the locally stored database and will only upload the changes that have been made to optimize low-speed internet access.

FEATURES
DESIGNED FROM THE GROUND UP WITH ITS END USERS’ NEEDS IN MIND
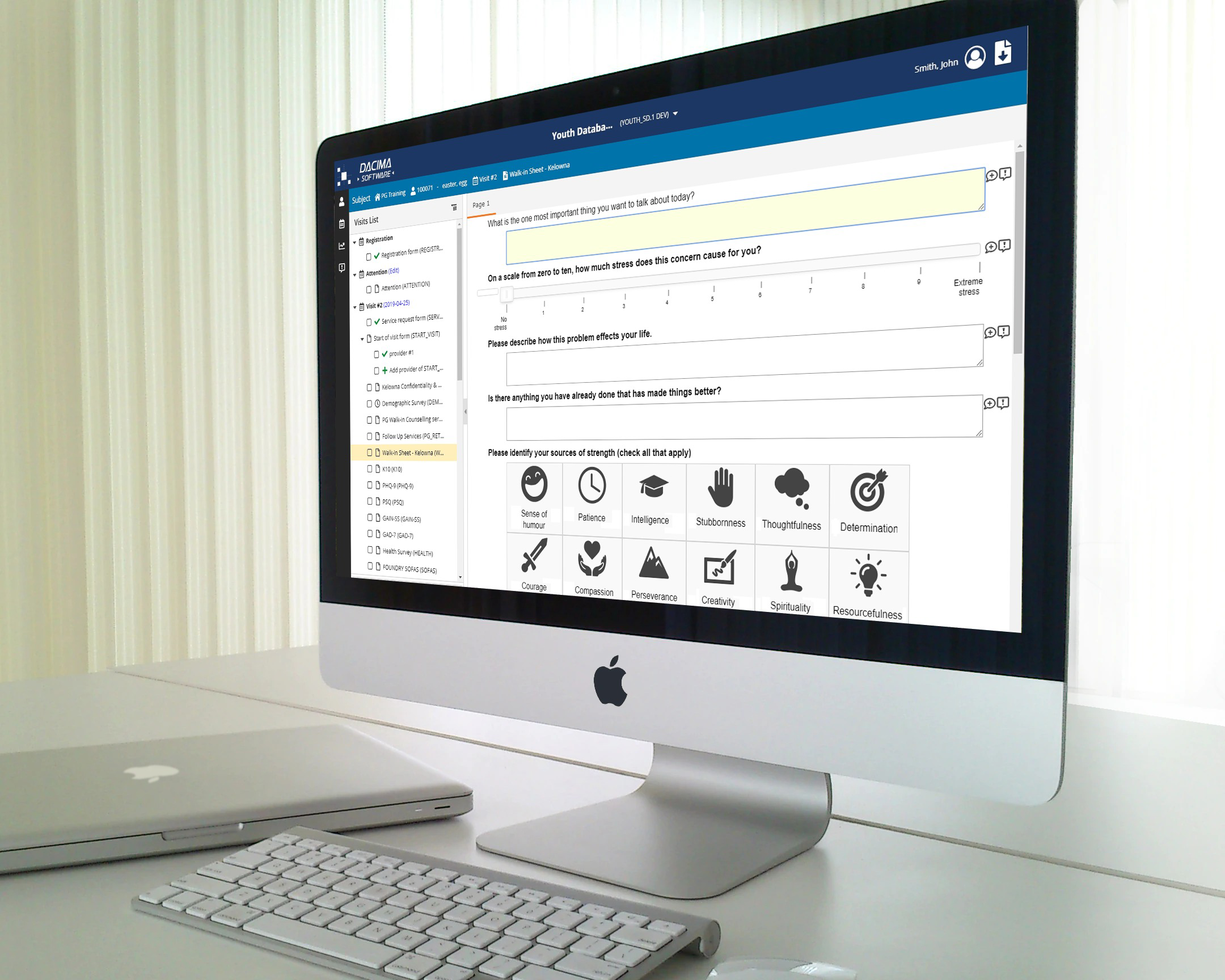
Dacima Clinical Suite EDC offers an integrated eSource solution. The platform includes digital Direct Data Entry (DDC), electronic Patient Reported Outcomes (ePRO), electronic Clinical Outcomes Assessment (eCOA), electronic Diaries (eDiary), eConsent (eCON) and other types of data collected during the trial. Data is entered into the system in real-time, eliminating the need for later transcription of data from paper copies, saving time and reducing errors.
Powerful Web Database Development
Allows you to develop databases without the need for computer programming expertise and access them through any web browser.
Flexible, Commercial Technology
Activating or deactivating features, the software can be easily tailored for different research study designs, such as randomized clinical trials, observational epidemiological studies, patient registries, patient interviews or administrative databases.
Auditable and Fully Compliant
The system complies with FDA CFR 21 Part 11 regulations, can be used for data collection in clinical research such as for randomized clinical trials, and is compliant with Good Clinical Practices (GCP) and regulatory requirements (EMEA, PMDA, FDA and ICH).
Reporting Made Easy
In addition to built-in standard reports, Dacima EDC includes a powerful query builder. Extract specific data and create custom reports without the need for programming.
Achieve More Accurate Results Faster
Data validation and quality control features for validation checks, skip patterns, formula within form or across different forms. Dynamic logic for form activation/deactivation to trigger forms based on the data values entered alongside validation controls executed in real time.
Forms That Are Easy to Use and Understand
Embed subforms within main forms creating more fluid and user-friendly experience, simplifying the data entry process, and increasing data entry efficiency. Create tabular display of data entry items and split your form into sections using the panels feature. Create forms that closely resemble your paper questionnaires or Case Report Forms.

DIGITAL GIFT INTEGRATION
REWARD RESPONDANTS WITHOUT JEOPARDIZING ANONYMITY
Our EDC integrates with Rybbon offering best-in-class security and anonymous delivery, so you can offer incentives with no worries. Our Recipient Data Masking option means you can reward clinical trial participants without access to their identities.
Use with international studies as rewards can be redeemed in more than 150 countries around the globe.

